Have you always wanted to know how a medicine is made? Thanks to this file, find all the stages of the drug cycle, which make it possible to ensure its effectiveness and its harmlessness, from its conception to its distribution.

The stages of the drug cycle
Obtaining a new drug is a long-term step that involves a large number of players in both the medical and administrative fields. The time required between the discovery of a molecule promising and the arrival of the drug on the market is around ten years.
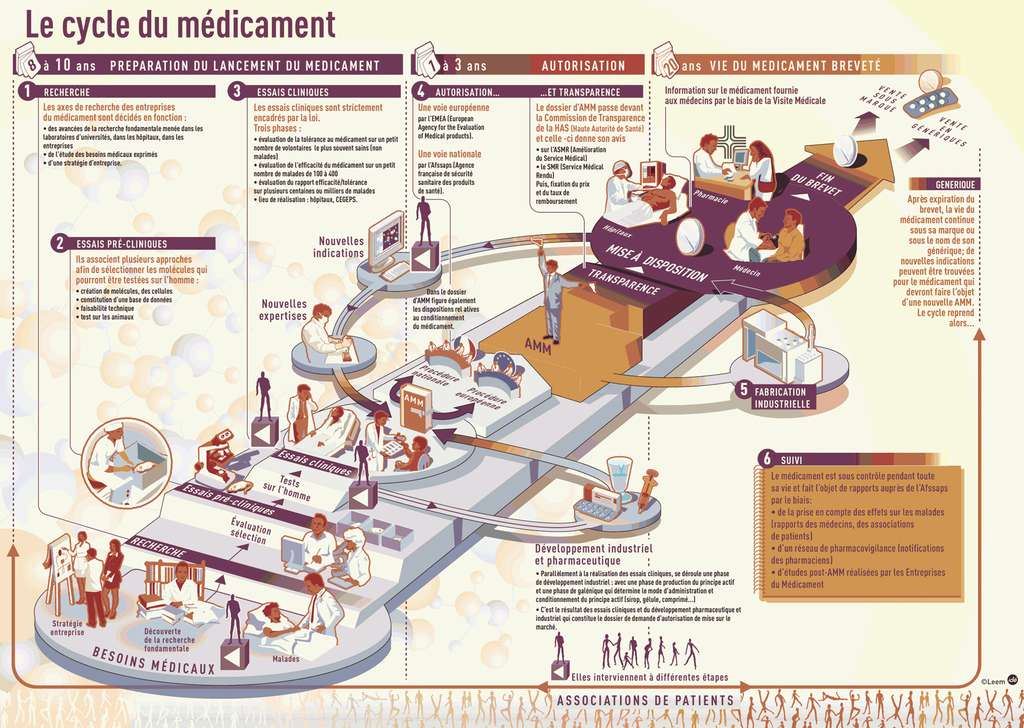
What research is needed to obtain potentially useful molecules? What tests are performed before clinical tests made on Man? Who authorizes the marketing of medicines and under what conditions? What checks are carried out to make sure that the medicine fulfills its functions? Find all the answers to these questions on the pages of this file.
To note
After the case of Picks, the organizations managing the marketing of medicines have been restructured. In this file, we quote Philippe Lechat, director from 2007 to 2012 of the evaluation of drugs and biological products at the French Agency for Health Safety of Health Products (Afssaps). In 2012, theNational Agency for the Safety of Medicines and Health Products (ANSM) has replaced Afssaps. ANSM has fully taken over the rights, missions and obligations of Afssaps and has been given a new organization and a completely revised mode of governance. ANSM assesses the quality, effectiveness, health risks and benefits linked to medicines and health products by ensuring the control of medicines in the laboratory as well as the marketing authorizations (MA).
Read also on ABSMARTHEALTH
Discover more future smart health innovation/ with AB SMART HEALTH








